
When the University of Southern Indiana Art and Design Department began planning for Solarpalooza, it was immediately clear that cyanotype art— which involves using the sun to create beautiful prints—would be a perfect activity.
Cyanotype art is an archaic photographic printing process that produces a cyan-blue print. Known for its signature Prussian blue color, cyanotype dyes are made by combining two premade solutions. When exposed to ultraviolet light—a spectrum that includes sunlight—a chemical reaction occurs, and a beautiful blue piece of art is created.
On the day of the eclipse, the Art and Design Department had one of the premade solutions, containing potassium ferricyanate, but soon ran out of the other.
“This is when it got interesting. I sent two students to make more coating solution, and they promptly returned letting me know the box was missing a key ingredient,” says Rob Dickes, Assistant Professor of Photography and Digital Imaging. “I tried to come up with a workaround, including calling the company to see if we could make a solution from chemicals we had on hand. When no solution came forth, I remembered the chemistry department had a booth next to ours in the Arena.”
They needed a solution that contained ferric ammonium cytrate, but when the Chemistry Department's stockroom was found to be lacking this exact chemical, Dr. Jacob Lutter, Assistant Professor of Chemistry, and Chris Hogue, Laboratory Supervisor, had to improvise. By combining ferric ammonium sulfate and citrate salt in the proper amounts, they formed a solution that was similar to the missing solution.
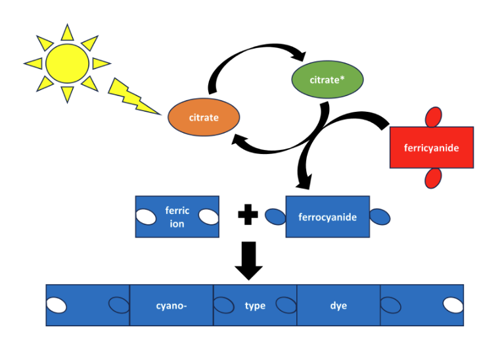 The goal of cyanotype dyes is essentially to create a color known as Prussian Blue. "Prussian Blue will form if there are two building blocks present, ferric ion and ferrocyanate ion. The cyanotype chemistry will make those building blocks and each chemical has a specific role," says Lutter. "The ferric ammonium citrate has two important parts—the ferric ion, one of the building blocks, and the citrate ion, which uses light to do some chemistry, to the reaction solution. The potassium ferricyanate supplies ferricyanate ions, a precursor for a building block, to the reaction solution. When combined, nothing will happen at first, but if light is introduced, the citrate will transform the ferricyanide into ferrocyanide—the actual building block. The ferric ion and ferrocyanide ion will then combine to form a solid compound that is the Prussian Blue dye."
The goal of cyanotype dyes is essentially to create a color known as Prussian Blue. "Prussian Blue will form if there are two building blocks present, ferric ion and ferrocyanate ion. The cyanotype chemistry will make those building blocks and each chemical has a specific role," says Lutter. "The ferric ammonium citrate has two important parts—the ferric ion, one of the building blocks, and the citrate ion, which uses light to do some chemistry, to the reaction solution. The potassium ferricyanate supplies ferricyanate ions, a precursor for a building block, to the reaction solution. When combined, nothing will happen at first, but if light is introduced, the citrate will transform the ferricyanide into ferrocyanide—the actual building block. The ferric ion and ferrocyanide ion will then combine to form a solid compound that is the Prussian Blue dye."
“It was amazing to see the Chemistry Department jump into action and create something the company that makes the chemistry could not even do. What Jacob and the Chemistry Department did was nothing short of miraculous,” says Dickes.